Describe the Periodic Trends for Electron Affinity.
Electron affinity increases upward for the groups and from left to right across periods of a periodic table because the electrons added to energy levels become closer to the nucleus thus a stronger attraction between the nucleus and its electrons. Electron affinity trend in group.
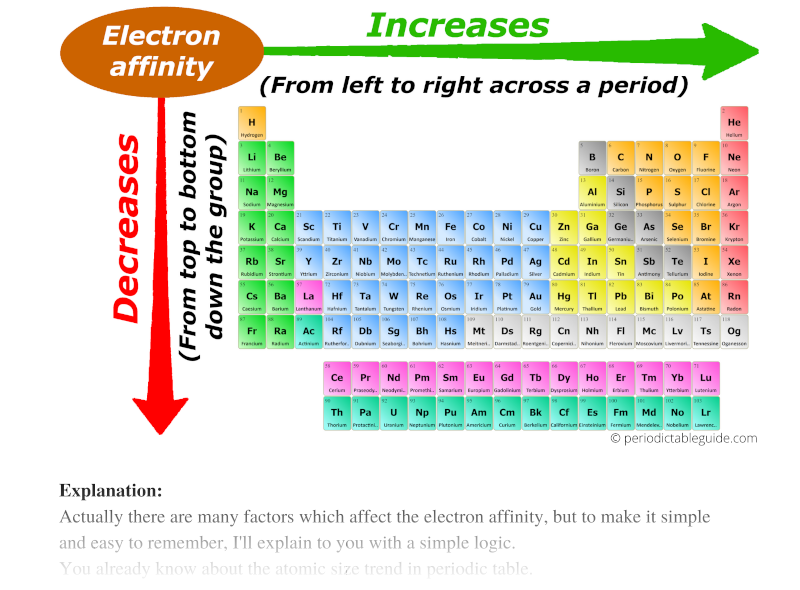
All Periodic Trends In Periodic Table Explained With Image
We tend to liken electron affinity to.
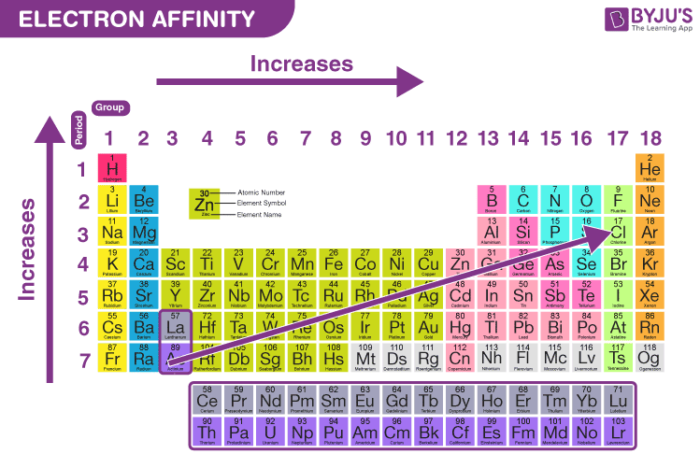
. Describe and explain trends in electron affinity across periods in the periodic table describe and explain exceptions or anomalies in trends seen throughout the periodic table predict the relative electron affinity of an element based on its position in. Electron affinity trend in a period. We review their content and use your feedback to keep the quality high.
Positively charged atoms are. Generally the atomic radius increases with element size atomic number. 100 3 ratings Electron affinity.
Which statement best describes the trend. Describe and explain trends in electron affinity across periods in the periodic table describe and explain exceptions or anomalies in trends seen throughout the periodic table predict the relative electron affinity of an element based on its position in the periodic table and neighboring elements. Answer link Truong-Son N.
Who are the experts. Chemists define electron affinity as the change in energy measured in units of kJmole experienced when an electron is added to a gaseous atom. Ionization energy and electron affinity also increase with increasing atomic number but primarily along the same row left to right of elements in the Periodic Table.
View the full answer. He often arrives late for work because as he tells his boss he spen. What are the trends and exceptions to the trends in electron affinity.
Yes it has an electron affinity value in minutes off energy and uh the units will be killing joke a whole colonies or any other units of energy. Ionization energy gets smaller as you move down the periodic table. Describe the periodic trends for electron affinity.
The pull on each electron in the outermost shell increases. This increases the opposite attracting force pulling the atom electron closer to the nucleus and then making the radius smaller protein pilling power. So how do the electron affinity very across the periodic table.
Electrons are added increasingly farther from the nucleus so the attractive force between the nucleus and electrons decreases. Check all that apply-The electron affinities of the elements in Group 17 are larger more negative than the elements in Group 1. It decreases as we move left to right in a periodic table becaus.
Electron affinity is increased from left to right in a period. As you move across the periodic table the effective nuclear charge increases. Here the former is closer to the nucleus and thus the size of the atom declines.
So um the electron affinity goes in the same direction as the atomic size. Electron Affinity Trend A Refresher On Atoms. Ben feels that his commitments to school family and his job are too demanding.
So its harder to remove those electrons. Electronegativity ionization energy electron affinity atomic radius melting point and metallic character. A As BI C K D CI E S.
The electron affinity trend describes the trend across the periodic table and describes how much energy in an atom is released or spent when an electron is added to a neutral atom or the energy change that occurs when an electron is added to a neutral atom. As one goes across a row of the periodic table a greater number of electrons and protons can be observed. The periodic trend for electron affinity values is not as consistent as for other trends.
Make a prediction about what trend you expect to see in electron affinity as you move down the periodic table. Protons neutrons and electrons. Electronegativity across a period.
Electron affinity generally increases from bottom to top within a group that is it goes to larger negative numbers and increases from left to right within a period. The Electron Affinity Trend. Check the correct box to describe the periodic trends in electronegativity.
This is due to the decreasing atomic sizes and encouraging the Nobel charges. 100 3 ratings Periodic trends are patterns that specifies many characteristic features of an element. I expect the electron affinity to decrease as I move down a group.
It is the distance between nucleus to the outermost shell of the atom. Atomic size periodic trend Group trend increases and periodic trend decreases Why do atoms get smaller as you move left to right in a period. These elements gain an electron energy shell and hence the atoms become larger.
More protons-more positive charge. As you move down the periodic table the radius of the valence shell. Electron configuration becomes more stable with added electrons as you move farther to the right.
Major periodic trends include. Atoms are made out of three different parts. Whats the reason alkali metals have the lowest and halogen have the highest value of electron affinity.
This process creates a negative ion. The overall trend across a period occurs because of increased nuclear attraction. Ions of atoms may have a net positive charge or a net negative charge.
Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element including its size and its electronic properties. Experts are tested by Chegg as specialists in their subject area. Identify which of the following atoms would have the greatest electron affinity.
The value of electron affinity usually becomes less negative on descending a group of the periodic table. Resulting anions more stable. Electron affinity decreases from upper to downward direction in a group.
Electron Affinity means how swiftly an atom accepts an. Reading List Question 21b of 22 Submit Describe and apply the periodic trends for electron affinity. This process differs from electronegativity which we define as the ability of an atom to attract an electron toward itself.
The halogens in Group 7A all have large negative electron affinities since they are only one electron away from having a noble gas configuration they easily accept another electron to generate stable halide anions.

What Is Electron Affinity Definition Trends Equation With Videos

High School Chemistry Electron Affinity Wikibooks Open Books For An Open World
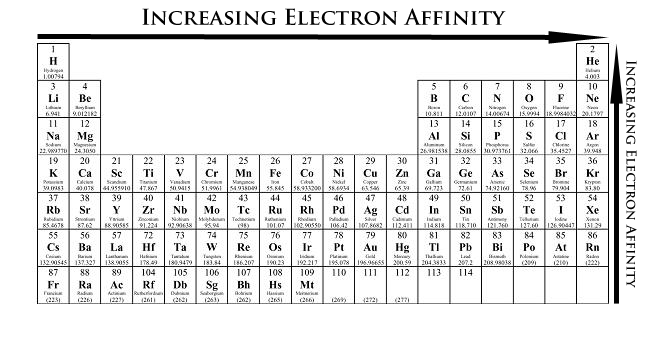
What Are The Periodic Trends For Atomic Radii Ionization Energy And Electron Affinity Socratic
Comments
Post a Comment